Pharsight Knowledgebase Server
The Pharsight Knowledgebase Server (PKS) is a database system for secure storage of study data and analyses. The PKS provides a secure data management system with complete auditing capabilities. The combination of the PKS and Phoenix provides an effective means to share source data, models, scripts and results among the members of a drug development team. The combination also supports compliance with the U.S. Food and Drug Administration’s 21 CFR Part 11 regulation.
Phoenix can work in conjunction with the Pharsight Knowledgebase Server (PKS) for secure storage and change tracking for data and analyses. This chapter covers the use of Phoenix as a PKS client: loading PKS data to Phoenix and saving data from Phoenix as PKS studies and scenarios. The PKS can also be accessed by way of a Web interface, which provides additional operations, and an XML API. The PKS functions and operations are covered in the Pharsight Knowledgebase Server User’s Guide.
This section contains the following topics:
The PKS information structure
Dosing
Setting up a PKS connection
PKS Browser
Creating a PKS study
PKS study properties
PKS study data
Detaching PKS objects from source
PKS Save and Save As options
Configuring default save options
Loading and exporting multiple PKS objects
Global Library objects
PKS Job Viewer
Phoenix is an object (text, chart, model, data) based application. Those objects fit into specific niches in the PKS data structure. PKS saves observations and related data as a PKS study. Analysis settings and output are added to the study as scenarios. Non-Phoenix documents, such as analysis output, reports, and graphics that are related to the study, can be loaded into the PKS and associated with a scenario.
The basic unit of information in the PKS is a study. A study starts with a collection of raw data that must include longitudinal observations, observation times and subject identifiers, and may include dosing and subject demographics. A study must have all of these data characteristics if data is mapped to PKS internal structures, but the study can contain any type of data if stored in the study library. Studies can be created with data mapped to the PKS data structures, or they can contain file-based data, maintained in the study library. As analyses are performed on these data, the analyses, including model settings and results, can be added as scenario(s) within the study.
A PKS study requires specific fields. First, a study must include at least one column containing subject identifiers. A collection of fields taken together can be used for subject identification. For example, first name, last name.
A study can include, but is not required to have, multiple columns of subject data: time-invariant, subject-specific information such as body weight or gender, that is, baseline covariates.
A study can include time-dependent observation and dosing data. Observation data can include records of concentration, measurements of blood pressure, heart rate, etc. Each data collection point can have multiple observations. In this case, a sampling variable is used to differentiate the samples.
If the observation data includes multiple, concurrent measurements for any subject, for example, multiple assays performed on a given sample or multiple samples during a given visit, the dataset must include a variable that differentiates the measurements, that is, values 1, 2, 3 for the 1st, 2nd and 3rd samples.
Because a study generally includes time-dependent observation data, a study must include relative nominal time, indicating the times at which observations and doses if included, were scheduled in the protocol. This is in contrast to relative actual time, representing the times that observations or doses really happened. Relative time is time elapsed since the start of the study, period or phase. If the data uses infusion dosing, the relative nominal end time must be identified as well. If only the Relative Nominal Time is defined in the dataset, a duplicate column is created for the actual times.
Note:To create a study that does include longitudinal observation data, include a dummy time column, with values for each row as placeholders, in the study.
Valid relative time units in PKS are: sec, min, hr, day, weeks, years. This restriction only applies to the nominal time, nominal end time, relative time, and relative end time. Other data elements can have any units (times, concentrations or other measurements).
When a study is created, information about the study can be entered in the Create Study dialog. This dialog includes information about the study: Study Name, Portfolio, Project, Indication, Study Type, Study Design, Compound, etc. The metadata provides ways to search and filter the data within the database. The metadata are saved with the study. See “Creating a PKS study” for more information about study metadata.
When an analysis is performed on a PKS study, the combination of source data, model, and results data can then be saved in the PKS as a scenario of the original study. A scenario can include a structural model, initial parameters, and/or statistical tests to be applied to a particular dataset for fitting or analysis, as well as any derived output from those tests or analysis. Scenarios are roughly comparable to the Phoenix concept of a project in that it is possible to save, in one place and under one name, related work: the data source, a model with model parameters, dosing, other model settings, and the analytical output. There is also a file created when the scenario is saved that contains all of the History entries for the scenario at the time of the save. Scenarios are associated with, that is, they are the children of a particular study data source, although it is possible to create further scenarios from a study or from an existing scenarios. See “Creating a Scenario” for more information.
Note:Loading a PKS scenario and submitting the workflow to the JMS for processing can prevent the scenario from being saved if the PKS middle-tier URL has changed since the scenario was last saved. If this occurs, run the scenario locally and save it to the PKS again.
The PKS can store documents, or library objects, such as study reports, SAS or NONMEM code files, or a graphic created by saving a Phoenix chart to an image file. Sets of documents can be associated with scenarios. See “Global Library objects” for more information.
Dosing information is a special case of study data. Dosing is generally saved as part of a PKS study and it is preferable to have the dosing data in the same worksheet as the observations, though it is not required.
If dose data is in a separate worksheet, it can be added to a study by loading it during study creation and its variables assigned as part of mapping the study variables. See “Creating a PKS study” for more information.
The dosing worksheet must contain, at a minimum, subject identifiers, dose amounts, and relative nominal dose times. It must contain the sort variables required to distinguish profiles. The worksheet can contain columns for more than one analysis scenario. Users can select which dose columns to use for a given scenario. See “Creating a Scenario” for more information.
To append dosing data to an existing PKS study, import a worksheet containing the dose information. Use the PKS Append/Update feature to map the new data columns to PKS study fields. See “Editing study data” for more information.
The PKS connection information must be provided before Phoenix can connect to PKS installations.
‘+
-
Click Add. The PKS Configuration dialog is displayed.
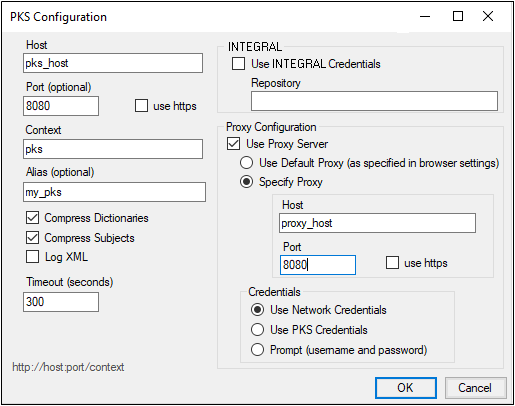
-
Enter the requested configuration properties.
-
Leave the Compress Subjects checkbox selected to improve performance by compressing study data per subject as it is transmitted to the PKS.
•Clearing the Compress Subjects checkbox is only recommended for diagnostic purposes.
•Selecting the Log XML checkbox is only recommended for diagnostic purposes.
-
To use your Integral login information, check Use Integral Credentials and enter the web location for the repository in the field.
-
To connect via proxy from the Phoenix PKS Plugin, configure the proxy information with the specific instance.
-
Check the Use Proxy Server checkbox.
If this checkbox is not checked, the controls inside the proxy configuration settings are disabled, and no proxy will be used. -
Select Default Proxy or click Specify Proxy to enter the Host and Port information to access a different proxy.
-
Three Credentials or authentication methods are available for passing credentials to the proxy server. Select one of the credential methods:
Use Network Credentials: The system passes the credentials used to access the network.
Use PKS Credentials: The user name and password specified for the PKS connection are passed to the proxy by the system. (Users who have also configured PKS with LDAP authentication may want to select this option.)
Prompt (username and password): The system prompts for the proxy user name and password. -
Click OK to save the new PKS instance.
-
Click OK to close the Preferences dialog.
Users can edit PKS instances after they are created. In the Instances page in the Preferences dialog, select a PKS instances and click Edit to edit it and Remove to remove it.
Users cannot edit or remove instances to which they are currently connected.
