The Model tab allows specifying the bioequivalence model.
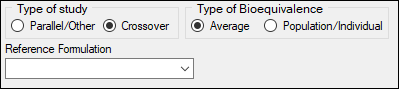
Under Type of study, select the Parallel/Other or Crossover option buttons to select the type of study.
Under Type of Bioequivalence, select the Average or Population/Individual option buttons to select the type of bioequivalence.
Crossover studies are the only study type permitted when population or individual are the types of bioequivalence being determined.
In the Reference Formulation menu, select the reference formulation or treatment.
The menu is only available after a formulation variable is mapped.
The Bioequivalence object automatically sets up default fixed effects, random effects, and repeated models for average bioequivalence studies depending on the type of study design: replicated crossover, nonreplicated crossover, or parallel. For more details on the default settings, see the “Average bioequivalence study designs” section.
For more information on population and individual bioequivalence, see the “Population and individual bioequivalence” section.