Managing Standard File Reference Titles
Many companies develop standard naming conventions for documents included in regulatory submissions. To support such standards, PUBLISH allows a publishing administrator to designate one or more standard titles that can be applied to newly added file references based on the section in which they are placed.
Pre-defined titles appear on the Titles tab as shown in the “Setting Titles” section.
One or more standard titles can be defined for each TOC section. The title marked as “Default” will be applied automatically when a file is added to that TOC section in any application. A publisher can modify or replace the standard title.
Possibilities for titles include:
• The file name of the physical file, corrected to remove the file extension, use title case and replace underscores or dashes with spaces. (For example, if a file named investigator-cv-for-study-1234.pdf is added, the title would be Investigator CV For Study 1234.) The <use file name> option is automatically available for each TOC section and cannot be deleted, although it need not be used.
• Any desired text, which can include properties applicable to that section. For example, a title has been added which includes a property ([Sequence ID]). When this title is applied to an actual file reference, the appropriate section number is automatically filled in (e.g., Cover Letter 0009).
The following figure shows how you can add properties to a standard title. Only those properties that are applicable to the section are shown. In this example, a standard title is being set for 3.2.P.1 Description and Composition of the Drug Product. Therefore, only general sequence properties and properties relating to the Drug Product section are shown. (Product is the product name used in 3.2.P and Sequence Product is the product name set up for the application and sequence.)
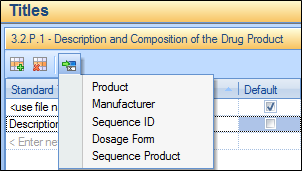
To add a new title, click in an empty row and type in the desired title. To add one or more properties, click the Insert  icon and choose from the dropdown list. If you want this to be the default file title (the title automatically applied when a new file reference is added) then check the Default check box (this will cause the existing default to be un-checked). When you are done, click the Enter The new title will become available in any application being edited.
icon and choose from the dropdown list. If you want this to be the default file title (the title automatically applied when a new file reference is added) then check the Default check box (this will cause the existing default to be un-checked). When you are done, click the Enter The new title will become available in any application being edited.
Existing titles can be modified by selecting their text and typing in new values and any standard title (except for <use file name> can be deleted by clicking its row and clicking the Remove  icon. For study specific sections, standard titles can be added in two ways:
icon. For study specific sections, standard titles can be added in two ways:
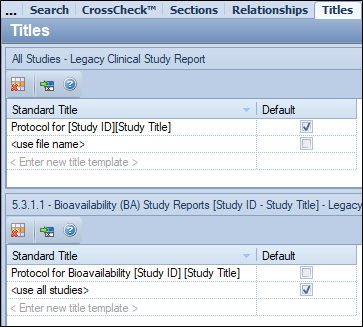
• The title can be added for all types of clinical or nonclinical studies. In the example below, the title “Protocol for [Study ID] [Study Title]” in the top pane will be available for any clinical study in any section of Module 5. A default value must be chosen in this section of the screen.
• The title can be added for a specific section such as “5.3.1.1 Bioavailability (BA) Study Reports”. In the example below, “Protocol for Bioavailability [Study ID] [Study Title]” in the bottom pane will be available only for studies under “5.3.1.1 Bioavailability (BA) Study Reports”. If a default value is chosen in this section of the screen, the default title will override the default title chosen in the general (top) section.