When the pharmacologic response takes time to develop and the observed response is not directly related to plasma concentrations of the drug a link model can be applied to relate the pharmacokinetics of the drug to its pharmacodynamics. Phoenix contains a group of indirect pharmacodynamic response (IPR) models that differ from other models in Phoenix in that they use a PK model to predict concentrations, and then use these concentrations as input to the indirect PD response model.
Additional details regarding the IPR models in Phoenix can be found in the following articles:
Jusko (1990). Corticosteroid pharmacodynamics: models for a broad array of receptor-mediated pharmacologic effects. J Clin Pharmacol 30:303.
Dayneka, Garg and Jusko (1993). Comparison of four basic models of indirect pharmacodynamic responses. J Pharmacokinet Biopharm 21:457.
Model 51: Inhibition of input (ind inhib input).
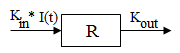
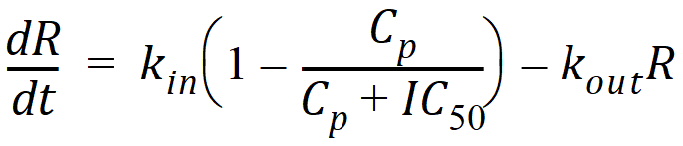
Estimated parameters: Kin Kout IC50
Model 52: Inhibition of output (ind inhib output).
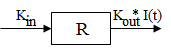
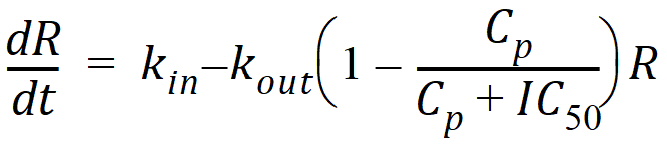
Estimated parameters: Kin Kout IC50
Model 53: Stimulation of input (ind stim input).

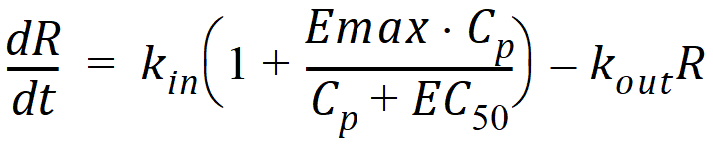
Estimated parameters: Kin Kout EC50 Emax
Model 54: Stimulation of output (ind stim output).
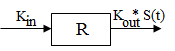
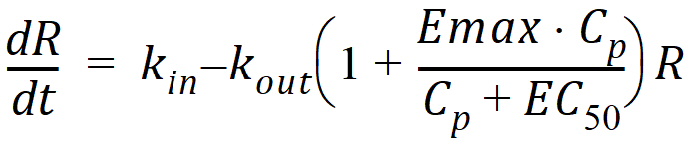
Estimated parameters: Kin Kout EC50 Emax
Model notation
R: Measured response to a drug.
kin: The zero-order constant for the production of response.
kout: The first-order rate constant for loss of response.
Cp: Plasma concentration of a drug.
Ce: Drug concentration at the effect site.
IC50: Drug concentration required to produce 50% of the maximal inhibition.
Emax: Maximum drug effect.
EC50: Concentration in plasma that achieves 50% of predicted maximum effect in an Emax model.