Evaluating individual and population bioequivalence example
Phoenix can handle a wide variety of model designs suitable for assessing individual and population bioequivalence, including:
TRTR/RTRT/TRRT/RTTR
TT/RR/TR/RT
TRT/RTR/TRR/RTT
TRRTT/RTTRR
TRR/RTR/RRT
RTR/TRT
TRR/RTT/TRT/RTR/TTR/RRT
TRRR/RTTT
TTRR/RRTT/TRRT/RTTR/TRRR/RTTT
where T = Test formulation and R = Reference formulation.
Note: Each sequence must contain the same number of periods. For each period, each subject must have one measurement.
A bioequivalence example, included as part of the “Testing the Phoenix installation” section, shows results for an RTR/TRT design. This example demonstrates an analysis of a TT/RR/TR/RT design.
Note: The completed project (Bioequivalence_IndPop.phxproj) is available for reference in …\Examples\WinNonlin.
Set up the population/individual model
Create a project called Bioequivalence_IndPop.
Import the file …\Examples\WinNonlin\Supporting files\TT RR RT TR.DAT.
Notice that the number of subjects is not the same in each sequence group. TT, RR, TR, and RT each have 4 subjects, whereas RT has 5.
Right-click TT RR RT TR in the Data folder and select Send To > Computation Tools > Bioequivalence.
In the Model tab below the Setup panel, select Population/Individual in the Type of Bioequivalence area.
Map the columns to the contexts as follows:
AUC to Dependent.
Leave Sequence mapped to Sequence.
Leave Subject mapped to Subject.
Leave Period mapped to Period.
Leave Formulation mapped to Formulation.
In the Model tab, make sure that:
Crossover is selected in the Type of study area. Crossover studies are the only permitted type for Population/Individual bioequivalence analysis.
Population/Individual is set as the Type of Bioequivalence.
R is selected as the Reference Value.
In the Fixed Effects tab, make sure that Ln(x) is set as the Dependent Variables Transformation.
The values will be log-transformed before the analysis.
Select the Options tab and enter 95 as the Confidence Level.
Execute and view the Population/Individual model results
Execute the object.
Select the Population Individual worksheet in the Results list.
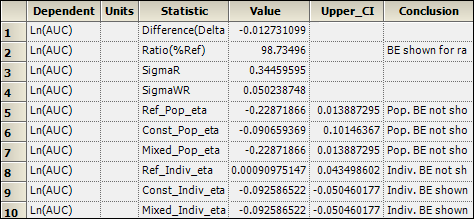
Inspect the results for mixed scaling. For population bioequivalence, the upper limit is 0.014 > 0, and therefore population BE has not been shown. For individual bioequivalence, the upper limit is –0.05 < 0, and so individual BE has been shown.
Compare average bioequivalence
Right-click the Bioequivalence object in the Object Browser and select Copy
Right-click Workflow in the Object Browser and select Paste.
In the Model tab of the copied object, select Average as the Type of Bioequivalence.
Make sure that:
Crossover is selected as the Type of study.
R is selected as the Reference Formulation.
In the Fixed Effects tab, make sure that:
Sequence+Formulation+Period appears in the Model Specification field.
Ln(x) is selected as the Dependent Variables Transformation.
Select the Variance Structure tab.
In the Random 1 sub-tab, make sure that:
Formulation appears in the Random Effects Model field.
Subject appears in the Variance Blocking Variables field.
Banded No-Diagonal Factor Analytic(f) is selected as the Type.
2 is in the Number of factors field.
In the Repeated sub-tab, make sure that:
Period appears in the Repeated Specification field.
Subject appears in the Variance Blocking Variables field.
Formulation appears in the Group field.
Execute and view the average bioequivalence results
Execute the object.
Using the model for average bioequivalence on replicated crossover designs resulted in a 90% lower interval of 87.277% (CI_90_Lower) and a 99.715% upper interval (CI_90_Upper) for the ratio of average AUC. Therefore, it can also be concluded that average bioequivalence is achieved. This is not always the case. Data can pass individual BE and fail average BE, and data can also pass average BE and fail individual BE.
This concludes the Bioequivalence individual/population evaluation example.