Central compartments can be connected to other PK compartments, including at least one elimination compartment (unless no drug leaves the body), by using a PK flow. Any number of central and peripheral compartments can be connected, with one exception: if a set of PK compartments using volume parameters are connected by PK flows parameterized in microconstants, there can be only one central compartment.
Click  in the Model toolbox.
in the Model toolbox.
Or
Select Insert > Compartment > Central from the right-click menu.
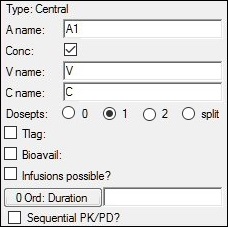
With the compartment selected, modify the default dosing variable name in the A name field, if desired.
Typically, A1 is used for intravenous input and Aa is used for extravascular input.
Check the Conc box if the compartment has concentration or volume parameters; uncheck it if there are no concentration or volume parameters.
The Conc checkbox controls the parameterization of the compartment. Selecting it adds the volume parameter V and the concentration parameter C to the model. When the checkbox is cleared, the compartment has no volume or concentration parameters, and can be useful for certain compartmental PD models.
In the fields for the other structural parameters, enter names for each parameter.
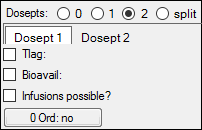
Select a Dosepts option (0, 1, 2, or split) to specify the number of dose points used by the central compartment. Select split to split dosing into the central compartment between two dose points.
Dose points can be defined for any PK compartment. The time(s) and amount(s) of doses are defined in the dataset. Each drug can have multiple formulations. For example, a drug can have one formulation dosing into the central compartment (IV) and another formulation dosing into an absorption compartment (oral or intramuscular). A drug model can include any number of different formulations and different drugs.
Note: Option 2 requires the dosing data to be in separate columns in the input dataset. Option split requires the data to be in the same column.
If 1, 2, or split dose points are selected, time delay parameters, bioavailability expressions, and infusions can be added and zero-order absorption options specified. For 2 or split, these parameters can be set for each dose point by selecting the Dosept 1 and Dosept 2 tabs.
Check the Tlag box to add a time delay parameter to the model. Enter the time lag expression in the field.
Check the Bioavail box to measure bioavailability in the central compartment. Enter the bioavailability expression in the field.
Bioavailability expressions can be entered as a numerical value or a parameter. For example, if 0.5 is entered, half the drug goes into the compartment.
To estimate bioavailability, a parameter must be used instead of a number. For example, type F (fraction of dose absorbed parameter) in the Bioavail field to estimate drug availability. If new parameter names are used in the expression, these will have to be inserted into the model. See the “Parameter block” section for Parameter block usage instructions.
To estimate bioavailability using multiple dosing routes, then a second parameter like 1-F can be entered in the Dosept 2 tab. Using multiple dosing routes and bioavailability parameters can be useful when modeling first- and second-pass absorption rates.
Check the Infusions possible? box if the dosing uses infusions.
A dosing parameter rate, such as Aa Rate or A1 Rate, is added to the contexts in the Main Mappings panel.
A Duration? checkbox is also added in the Structure tab. Checking this box causes the context A1 or Aa Rate to change to A1 or Aa Duration.
Click 0 Ord: No multiple times to toggle through the zero-order options:
No: There is no zero-order input.
Rate: The drug is introduced into the system at a constant rate. Enter the zero-order rate expression in the field, as either a parameter or a numeric value.
Duration: The drug is introduced over a finite period of time, or duration. Enter the zero-order duration expression in the field, as either a parameter or a numeric value.
Check the Sequential PK/PD? box if the PK model is part of a PK/PD model that is being fitted sequentially. This will freeze the PK portion of the model and turn its random effects into covariates. See the “Sequential PK-PD population model fitting” section for more information.