Population bioequivalence (PBE) criteria are:
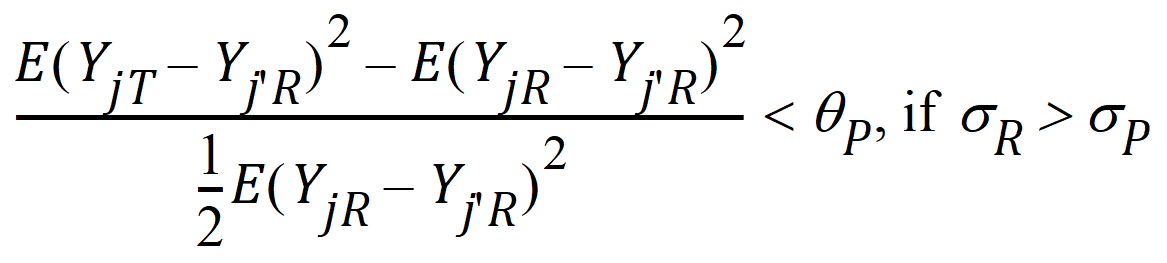
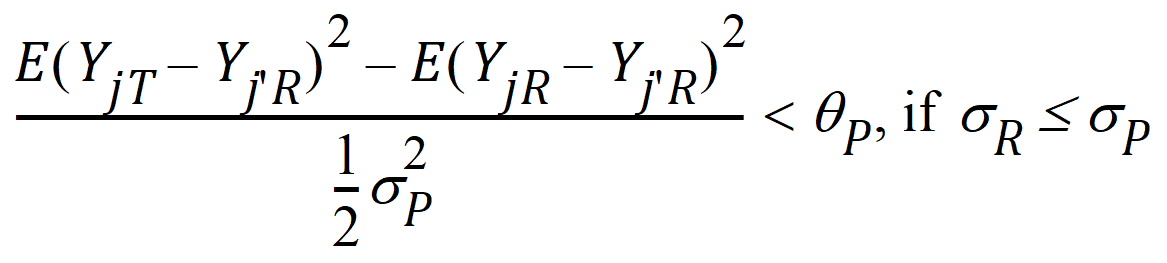
where sP defaults to 0.2 and qP=(ln(1 – PercentReference)+eP) /sP2. The default value for PercentReference is 0.20. In the Bioequivalence object, sP is called the Total SD standard. sR2 is computed by the program and is the total variance of the reference formulation, i.e., the sum of within- and between-subject variance. The criteria take the linearized form:
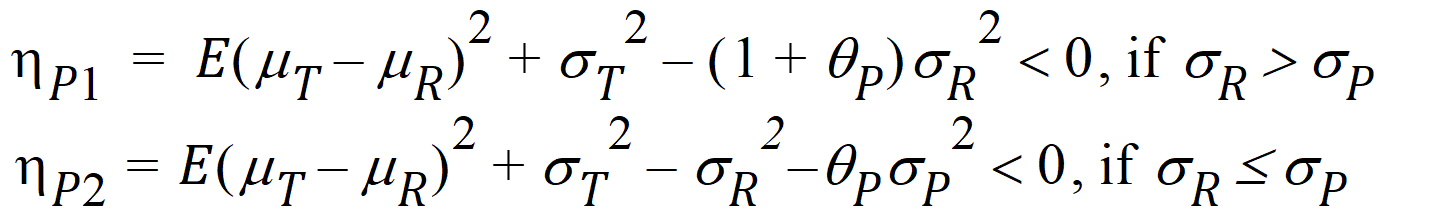 [
[
Individual bioequivalence (IBE) criteria are:
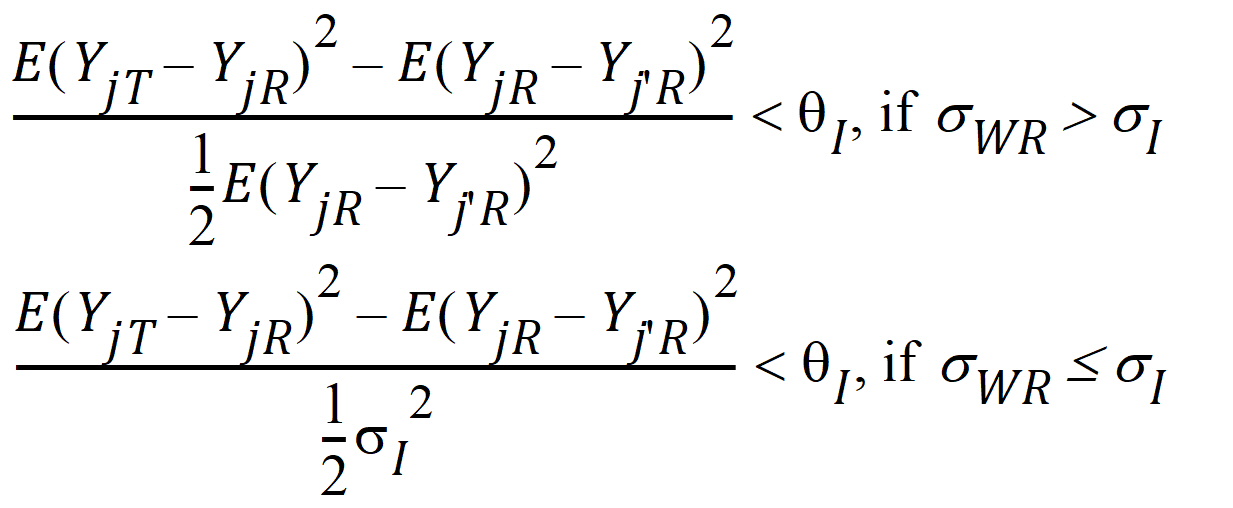
where sI defaults to 0.2 and qI=(ln(1 – PercentReference)+eI) /sI2. The default value for Percent Reference is 0.20, and the default value for eI is 0.05. In the Bioequivalence object, sI is called the within-subject SD standard. sWR is computed by Phoenix, and its square, sWR2, is the within-subject variance of the reference formulation.
The IBE criteria take the linearized form:
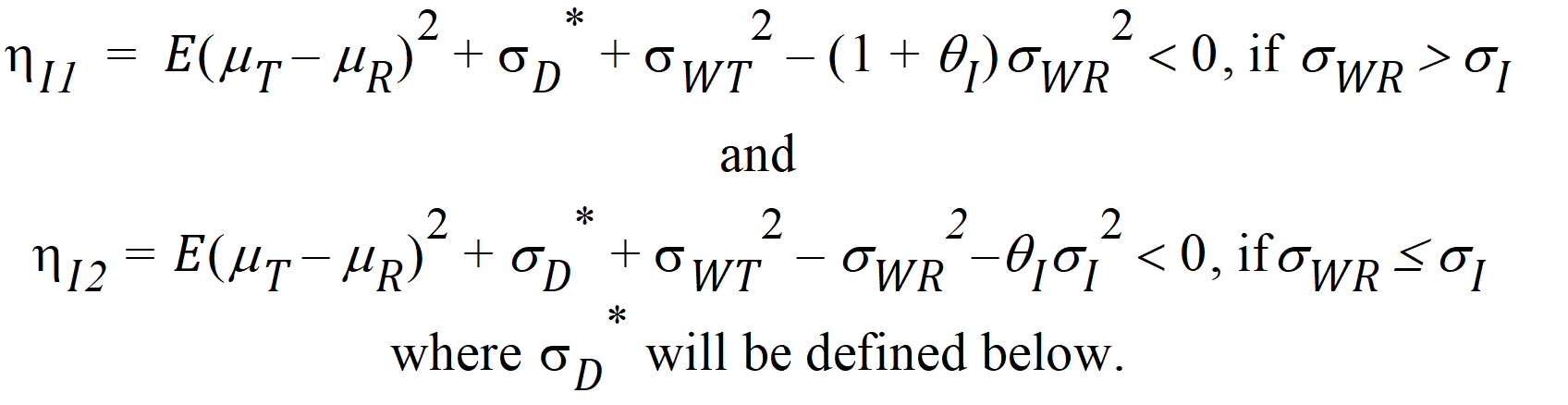
For reference scaling, use hP1 or hI1. For constant scaling, use hP2 or hI2. For mixed scaling, use one of the following.
Population
If sR > sPv, use hP1
If sR £ sP, use hP2
Individual
If sWR > sI, use hI1
If sWR £ sI, use hI2
If the upper bound on the appropriate h is less than zero, then the product is bioequivalent in the chosen sense. The interval is set in the Bioequivalence object’s Options tab. The method of calculating that upper bound follows.