PK Submit supports the automatic generation of a complete electronic PK regulatory submission package, including all necessary CDISC domains, during the normal process of setting up and executing an NCA.
The example below shows the basic steps to generating SEND 3.1 formatted submission-ready files.
See “SDTM example” for generating SDTM 3.2 formatted submission-ready files.
This example uses the input of a PC.xpt and an EX.xpt file. Using PK Submit, a full set of CDISC domains in SEND 3.1 format will be generated and an NCA project will be created and set up to use the domains. At the end of the example, you will have a complete set of files for the study that are ready for electronic submission.
The major steps in this example include:
•Create files required for submission
Create the PC domain
Specify the dataset for the PC domain
-
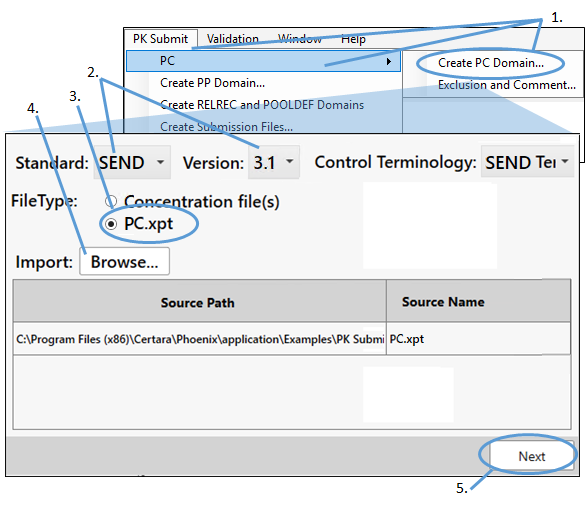
Select PK Submit > PC > Create PC Domain from the Phoenix menu.
-
In the PK Submit dialog, make sure the Standard and Version menus are set to SEND and 3.1, respectively.
-
Select PC.xpt as the FileType.
-
Click Browse, navigate to <Phoenix_install_dir>\application\Examples\PK Submit\Supporting files\Example1, select PC.xpt, and click Open.
-
Click Next.
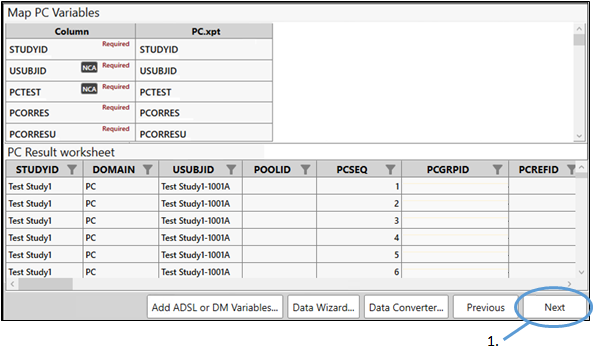
Map the variables
The next step in the process involves lining up variables in the input dataset with those in the PC domain. The variables are marked as either being required for the PC domain creation or optional. The example PC.xpt file we are using includes variables that are recognized by PK Submit as PC variables and, therefore, they are automatically mapped. There is no need for us to perform any manual mapping of the dataset variables to the PC variables.
Variables that are not required for the PC domain but are needed for the NCA can also be mapped at this point, such as Dose Amounts, Dose Units, and Routes of Administration. For this example, we will map these items in a later step.
Note:If a variable is manually mapped, the database installed with the application will remember the mapping and automatically map that variable the next time it is encountered.
All required variables must be mapped to complete the process. Our example input file contains all of the variables that are required for the PC domain so we can proceed to the next step.
-
Click Next.
During the PC domain creation process, PK Submit checks the data and will warn you of errors that will occur during the validation step. For example, if the USUBJID does not contain the studyID string, which is required for compliant PC domain, you will see a message and be given the option to have the PK Submit concatenate the study ID with the USUBJID to bring it into compliance.
Select NCA keys
-
For this example, we will use the default NCA keys. Click Next.
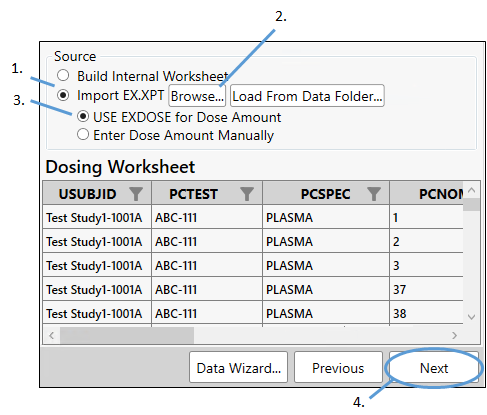
Merge dosing with concentration information
Dosing and concentration information can be merged by mapping the appropriate variables in the previous step, by entering values directly in the table, or by loading an EX.XPT file. For this example, we will load an example file.
-
Select the Import EX.XPT option.
-
Select the Use EXDOSE for Dose Amount option.
-
Click Browse, navigate to <Phoenix_install_dir>\application\Examples\PK Submit\Supporting files\Example1, select EX.xpt and click Open.
-
Click Next.
If the merge is performed successfully, PK Submit will tell you the number of rows of data were imported. Click OK.
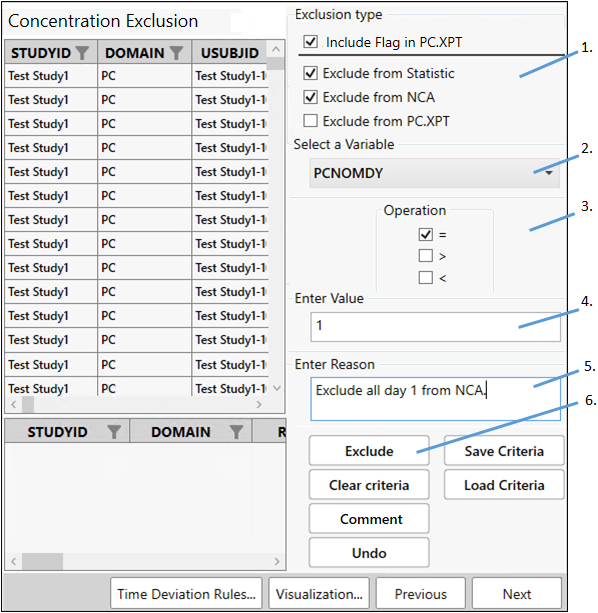
Exclude data
Set up a filter to exclude all day 1 data from the NCA analysis.
-
Check the Exclude from Statistic and Exclude from NCA boxes.
-
From the Select a Variable pulldown, scroll down the list and choose PCNOMDY.
-
Check the box for = under Operation.
-
In the Enter Value field, type 1.
-
Type the following in the Enter Reason or Comment field:
Exclude all day 1 from NCA. -
Click Exclude.
Rows that meet the criteria (row 1, in this example) will be shaded pink to indicate that they are marked for exclusion.
Add a comment
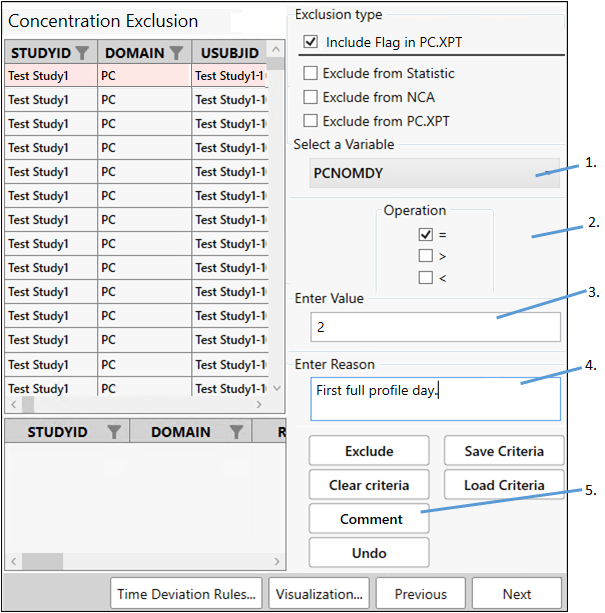
On the same Concentration Exclusion page, add a comment about the data collected on day 2.
-
From the Select a Variable pulldown, scroll down the list and choose PCNOMDY again.
-
Check the box for = under Operation.
-
In the Enter Value field, type 2.
-
Type the following in the Enter Reason or Comment field:
First full profile day. -
Click Comment.
-
Click Next.
Adding comments generates a CO domain.
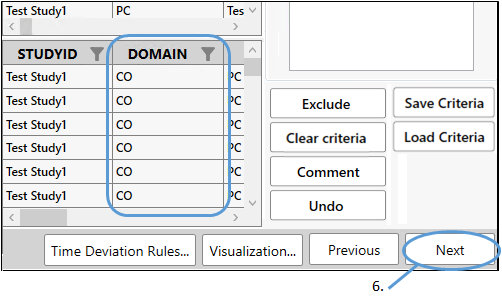
Finish setting up NCA
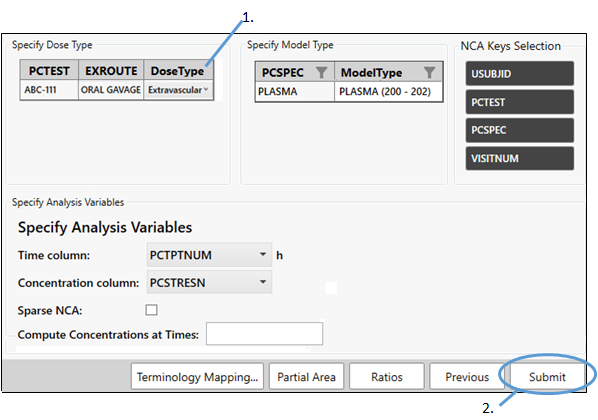
-
Select Extravascular from the Dose Type pulldown.
-
Click Submit.
The Terminology Mapping button will show how the terminology for specimen and units are being mapped. As mentioned earlier in the example, this mapping is remembered by the database and only needs to be mapped once. However, the complete terminology list is always available through the Controlled Terminology Mapping dialog for you to select from.
The PC domain is complete and a Phoenix project is automatically created, with a workflow that has an NCA object preconfigured according to specifications made in PK Submit.
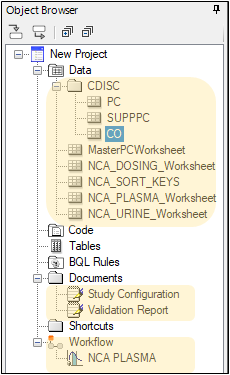
Create the PP domain
The PP domain can be generated from an NCA, typically the Final Parameters output worksheet. The NCA object set up by PK Submit is ready to execute. Unless you want to review the slope selector, you can simply run the NCA.
Specify the data for the PP domain
-
Rename the project as Example1.
-
Execute the NCA object.
-
Select PK Submit > Create PP Domain from the Phoenix menu.
-
Click OK.
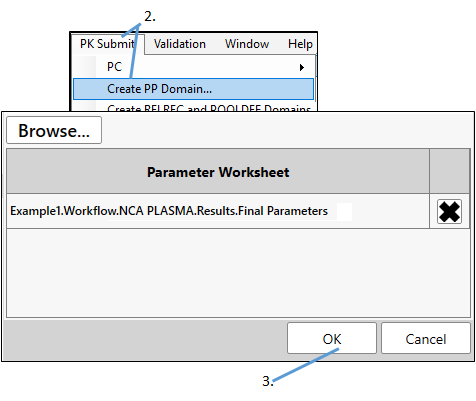
PK Submit will automatically select the Final Parameters result worksheet when there is an open project with an executed NCA object available (such as in this example). Otherwise, use the Browse button.
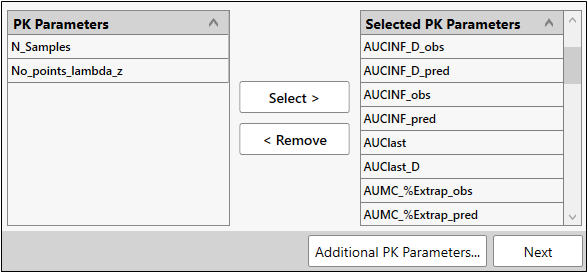
Select PK parameters
-
Using the Select > and < Remove buttons, move all of the parameters to the Selected PK Parameters list except for N_Samples and No_points_lambda_z.
-
Click Next.
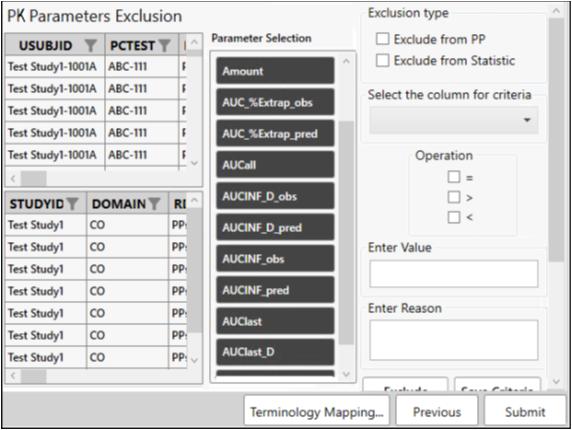
Exclude parameters
For this example, we will not set up any exclusion criteria.
-
Click Submit.
The PP domain is created.
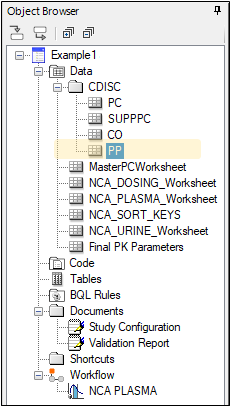
Create RELREC domain
-
Select PK Submit > Create RELREC and POOLDEF Domains from the Phoenix menu.
Since this was not a sparse study, there will not be a POOLDEF domain.
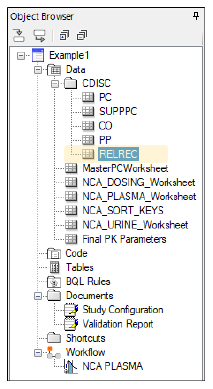
Create files required for submission
-
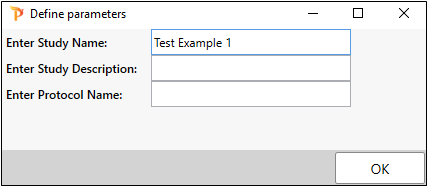
Select PK Submit > Create Submission Files from the Phoenix menu.
-
Enter Test Example 1 as the Study Name.
-
Click OK.
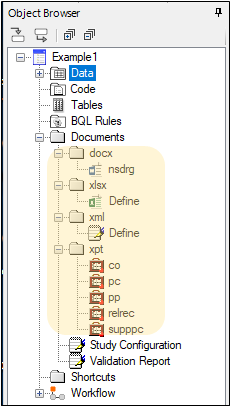
The Study Data Reviewer’s Guide is created and stored in the Documents > docx subfolder of the Object Browser. The template for this document is available on the PhUSE website. PK Submit automatically populates the document with most of the information needed for submission.
The Define.xlsx file is created and stored in the Documents > xlsx subfolder. This Excel-formatted file contains all of the information you find in the Define.xml file for the study in the Documents > xml subfolder.
-
Select PK Submit > Export Submission Files from the Phoenix menu.
-
In the file browser, select the location in which to save the files and click OK.
In the following image, a folder named Example1 was selected.
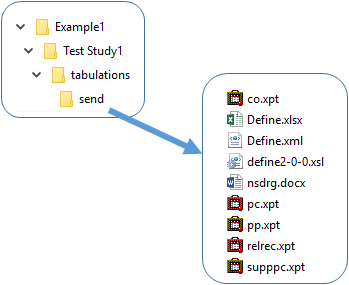
The study information is ready for electronic submission and the process is complete.
