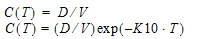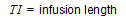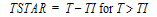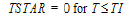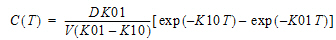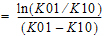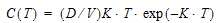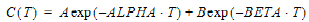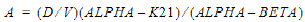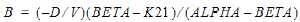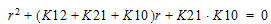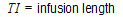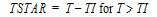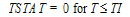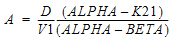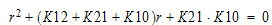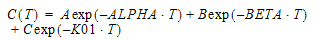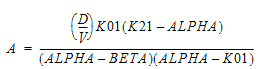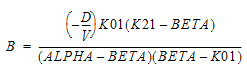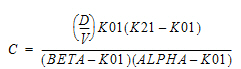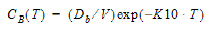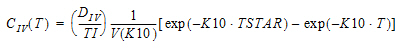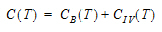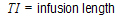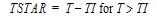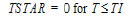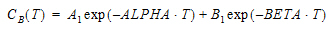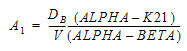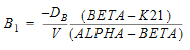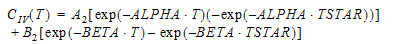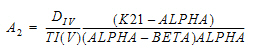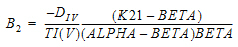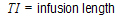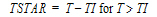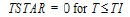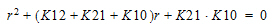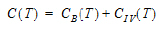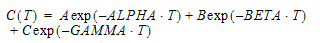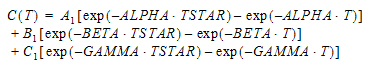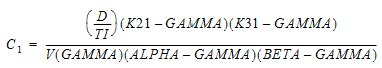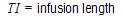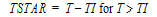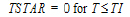Phoenix includes nineteen pharmacokinetic (PK) models. Each is available in the PK Model object.
|
Model |
Input |
Number of compartments |
Parameterization |
Lag time |
Elimination rate |
|
IV-bolus |
1 |
- |
no |
1st order |
|
|
IV-infusion |
1 |
- |
no |
1st order |
|
|
1st order |
1 |
- |
no |
1st order |
|
|
1st order |
1 |
- |
yes |
1st order |
|
|
1st order |
1 |
K10=K01 |
no |
1st order |
|
|
1st order |
1 |
K10=K01 |
yes |
1st order |
|
|
IV-bolus |
2 |
micro |
no |
1st order |
|
|
IV-bolus |
2 |
macro |
no |
1st order |
|
|
IV-infusion |
2 |
micro |
no |
1st order |
|
|
IV-infusion |
2 |
macro |
no |
1st order |
|
|
1st order |
2 |
micro |
no |
1st order |
|
|
1st order |
2 |
micro |
yes |
1st order |
|
|
1st order |
2 |
macro |
no |
1st order |
|
|
1st order |
2 |
macro |
yes |
1st order |
|
|
IV-bolus plus IV-infusion |
1 |
micro |
no |
1st order |
|
|
IV-bolus plus IV-infusion |
2 |
micro |
no |
1st order |
|
|
IV-bolus plus IV-infusion |
2 |
macro |
no |
1st order |
|
|
IV-bolus |
3 |
macro |
no |
1st order |
|
|
IV-infusion |
3 |
macro |
no |
1st order |
Note:All models except models 15–17 accept multiple dose data.
The models shown in this section give equations for compartment 1 (central).
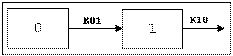
One-compartment with bolus input and first-order output.
|
|
|
|
Required constants |
Estimated parameters |
Secondary parameters |
Clearance estimated |
Clearance secondary |
|
N doses |
V is the volume |
AUC=D/V/K10 |
V |
AUC |
|
dose N |
K10 is the elimination rate |
K10 half-life |
CL |
K10 half-life |
|
time of dose N |
|
Cmax=D/V |
|
Cmax |
|
(Repeat for each dose) |
CL |
|
K10 |
|
|
AUMC |
|
AUMC |
||
|
MRT |
|
MRT |
||
|
Vss |
|
VSS |
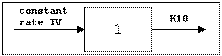
One-compartment with constant IV input, first-order absorption.
|
|
|
|
Required constants |
Estimated parameters |
Secondary parameters |
Clearance estimated |
Clearance secondary |
|
N doses |
V is the volume |
AUC=D/V/K10 |
V |
AUC |
|
dose N |
K10 is the elimination rate |
K10 half-life |
CL |
K10 half-life |
|
start time N |
|
Cmax=C(TI) |
|
Cmax |
|
end time N |
CL |
|
K10 |
|
|
(Repeat for each dose) |
AUMC |
|
AUMC |
|
|
MRT |
|
MRT |
||
|
Vss |
|
VSS |
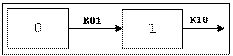
One-compartment with first-order input and output, no lag time.
|
|
|
|
Required constants |
Estimated parameters |
Secondary parameters |
Clearance estimated |
Clearance secondary |
|
N doses |
V_F |
AUC=D/V/K10 |
V_F |
AUC |
|
dose N |
K01 is the absorption rate |
K01 half-life |
K01 |
K01 half-life |
|
time of dose N |
K10 is the elimination rate |
K10 half-life |
CL_F |
K10 half-life |
|
(Repeat for each dose) |
|
CL_F |
|
K10 |
|
Tmax=time of Cmax |
|
Tmax |
||
|
Cmax is the max concentration |
|
Cmax |
One-compartment with first-order input and output with lag time.
Identical to Model 3, with an additional estimated parameter: Tlag=lag time.
One-compartment, equal first-order input and output, no lag time.
|
|
|
|
Required constants |
Estimated parameters |
Secondary parameters |
Clearance estimated |
Clearance secondary |
|
N doses |
V_F |
AUC=D/V/K |
V_F |
AUC |
|
dose N |
K is the absorption and |
K half-life |
CL_F |
K half-life |
|
time of dose N |
CL_F |
|
K |
|
|
(Repeat for each dose) |
|
Tmax is the time of Cmax=1/K |
|
Tmax |
|
Cmax is the max concentration |
|
Cmax |
One-compartment, equal first-order input and output with lag time.
Identical to Model 5, with an additional estimated parameter: Tlag=lag time.
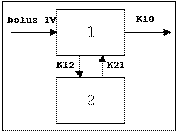
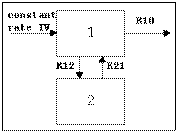
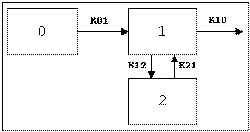
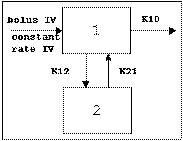
Two-compartment with bolus input and first-order output; micro-constants as primary parameters.
|
|
|
|
Required constants |
Estimated parameters |
Secondary parameters |
Clearance estimated |
Clearance secondary |
|
N doses |
V1 is Volume1 |
AUC=AUC=A/Alpha+B/Beta |
V1 |
AUC |
|
dose N |
K10 is the elimination rate |
K10 half-life |
CL |
K10 half-life |
|
time of dose N |
K12 is the transfer rate, 1 to 2 |
ALPHA |
V2 |
ALPHA |
|
(Repeat for each dose) |
K21 is the transfer rate, 2 to 1 |
BETA |
CLD2 |
BETA |
|
|
ALPHA half-life |
|
ALPHA half-life |
|
|
BETA half-life |
|
BETA half-life |
||
|
A |
|
A |
||
|
B |
|
B |
||
|
Cmax=D/V |
|
Cmax |
||
|
CL |
|
K10 |
||
|
AUMC |
|
AUMC |
||
|
MRT |
|
MRT |
||
|
Vss |
|
Vss |
||
|
V2 |
|
K12 |
||
|
CLD2 |
|
K21 |
Two-compartment with bolus input and first-order output; macro-constants as primary parameters.
As Model 7 with macroconstants as the primary (estimated) parameters. Clearance parameters are not available for Model 8.
|
Required constants |
Estimated parameters |
Secondary parameters |
|
|
stripping dose |
A |
AUC=A/ALPHA+B/BETA |
V1 |
|
N doses |
B |
K10 half-life |
CL |
|
dose N |
ALPHA |
ALPHA half-life |
AUMC |
|
time of dose N |
BETA |
BETA half-life |
MRT |
|
(Repeat for each dose) |
|
K10 |
Vss |
|
K12 |
V2 |
||
|
K21 |
CLD2 |
||
|
Cmax |
|
||
Two-compartment with constant IV input and first-order output; micro-constants as primary parameters.
|
|
|
|
Required constants |
Estimated parameters |
Secondary parameters |
Clearance estimated |
Clearance secondary |
|
N doses |
V1 is Volume1 |
AUC=D/V/K10 |
V1 |
AUC |
|
dose N |
K10 is the elimination rate |
K10 half-life |
CL |
K10 half-life |
|
start time N |
K12 is the transfer rate, 1 to 2 |
ALPHA |
V2 |
ALPHA |
|
end time N |
K21 is the transfer rate, 2 to 1 |
BETA |
CLD2 |
BETA |
|
(Repeat for each dose) |
|
ALPHA half-life |
|
ALPHA half-life |
|
BETA half-life |
|
BETA half-life |
||
|
A |
|
A |
||
|
B |
|
B |
||
|
Cmax=C(TI) |
|
Cmax |
||
|
CL |
|
K10 |
||
|
AUMC |
|
AUMC |
||
|
MRT |
|
MRT |
||
|
Vss |
|
Vss |
||
|
V2 |
|
K12 |
||
|
CLD2 |
|
K21 |
Two-compartment with constant IV input and first-order output; macro-constants as primary parameters.
As Model 9 with macroconstants as the primary (estimated) parameters. Clearance parameters are not available in Model 10.
|
Required constants |
Estimated parameters |
Secondary parameters |
|
|
N doses |
V1 |
K10 |
Cmax |
|
dose N |
K21 |
K12 |
CL |
|
start time N |
ALPHA |
K10 half-life |
AUMC |
|
end time N |
BETA |
AUC |
MRT |
|
(Repeat for each dose) |
|
ALPHA half-life |
Vss |
|
BETA half-life |
V2 |
||
|
A |
CLD2 |
||
|
B |
|
||
Two-compartment with first-order input, first-order output, no lag time and micro-constants as primary parameters.
|
|
|
|
Required constants |
Estimated parameters |
Secondary parameters |
Clearance estimated |
Clearance secondary |
|
N doses |
V1_F |
AUC=D/V/K10 |
V1_F |
AUC |
|
dose N |
K01 is the absorption rate |
K01 half-life |
K01 |
K01 half-life |
|
time of dose N |
K10 is the elimination rate |
K10 half-life |
CL_F |
K10 half-life |
|
(Repeat for each dose) |
K12 is the transfer rate, 1 to 2 |
ALPHA |
V2_F |
ALPHA |
|
K21 is the transfer rate, 2 to 1 |
BETA |
CLD2_F |
BETA |
|
|
|
ALPHA half-life |
|
ALPHA half-life |
|
|
BETA half-life |
|
BETA half-life |
||
|
A |
|
A |
||
|
B |
|
B |
||
|
CL_F |
|
K10 |
||
|
V2_F |
|
K12 |
||
|
CLD2_F |
|
K21 |
||
|
Tmaxa |
|
Tmax |
||
|
Cmaxa |
|
Cmax |
|
aEstimated for the compiled model only. |
Two-compartment with first-order input, first-order output, lag time and micro-constants as primary parameters.
As Model 11, with an additional estimated parameter: Tlag=lag time.
Two-compartment with first-order input, first-order output, no lag time and macro-constants as primary parameters.
As Model 11, with macroconstants as the primary (estimated) parameters. Clearance parameters are not available for Model 13.
|
Required constants |
Estimated parameters |
Secondary parameters |
|
|
Stripping dose |
A |
K10 |
BETA half-life |
|
N doses |
B |
K12 |
V1_F |
|
dose N |
K01 is the absorption rate |
K21 |
CL_F |
|
time of dose N |
ALPHA |
AUC=D/V/K10 |
V2_F |
|
(Repeat for each dose) |
BETA |
K01 half-life |
CLD2_F |
|
Note: C=–(A+B) |
K10 half-life |
Tmaxa |
|
|
|
ALPHA half-life |
Cmaxa |
|
|
aEstimated for the compiled model only. |
Two-compartment with first-order input, first-order output, lag time and macro-constants as primary parameters.
As Model 13, with an additional estimated parameter: Tlag=lag time.
One-compartment with simultaneous bolus IV and constant IV infusion.
|
|
|
Two-compartment with simultaneous bolus IV and constant infusion input; micro constants as primary parameters.
|
|
|
|
Required constants |
Estimated parameters |
Secondary parameters |
Clearance estimated |
Clearance secondary |
|
bolus dose |
V1 |
K10 half-life |
V1 |
K10 half-life |
|
IV dose |
K10 |
ALPHA |
CL |
ALPHA |
|
length of infusion (= TI) |
K12 |
BETA |
V2 |
BETA |
|
|
K21 |
ALPHA half-life |
CLD2 |
ALPHA half-life |
|
|
BETA half-life |
|
BETA half-life |
|
|
A |
|
A |
||
|
B |
|
B |
||
|
CL |
|
K10 |
||
|
V2 |
|
K12 |
||
|
CLD2 |
|
K21 |
Two-compartment with simultaneous bolus IV and constant infusion input and macro constants as primary parameters.
As Model 16 with macroconstants as the primary (estimated) parameters.
|
Required constants |
Estimated parameters |
Secondary parameters |
|
|
stripping dose |
A |
K10 |
BETA half-life |
|
bolus dose |
B |
K12 |
V1 |
|
IV dose |
ALPHA |
K21 |
CL |
|
length of infusion (= TI) |
BETA |
K10 half-life |
V2 |
|
|
|
ALPHA half-life |
CLD2 |
Clearance parameters are not available in Model 17.
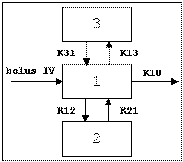
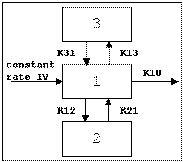
Three-compartment with bolus input, first-order output; macro constants as primary parameters.
|
|
|
|
Required constants |
Estimated parameters |
Secondary parameters |
|
|
stripping dose |
A |
Cmax |
GAMMA half-life |
|
N doses |
B |
V1 |
AUC |
|
dose N |
C |
K21 |
CL |
|
time of dose N |
ALPHA |
K31 |
AUMC |
|
(Repeat for each dose) |
BETA |
K10 |
MRT |
|
GAMMA |
K12 |
Vss |
|
|
|
K13 |
V2 |
|
|
|
K10 half-life |
CLD2 |
|
|
|
ALPHA half-life |
V3 |
|
|
|
BETA half-life |
CLD3 |
|
Clearance parameters are not available in Model 18.
Three compartment model with constant IV infusion; macro constants as primary parameters.
|
|
|
|
Required constants |
Estimated parameters |
Secondary parameters |
||
|
N doses |
V1 |
Cmax |
K10 half-life |
MRT |
|
dose N |
K21 |
K10 |
ALPHA half-life |
Vss |
|
start time of dose N |
K31 |
K12 |
BETA half-life |
V2 |
|
end time of dose N |
ALPHA |
K13 |
GAMMA half-life |
CLD2 |
|
(Repeat for each dose) |
BETA |
A |
AUC |
V3 |
|
|
GAMMA |
B |
CL |
CLD3 |
|
|
|
C |
AUMC |
|
-
A, B, and C are the zero time intercepts following an IV injection.
-
Clearance parameters are not available in Model 19.