The Michaelis-Menten models describe the relationship between the rate of substrate conversion by an enzyme to the concentration of the substrate. These kinetics are valid only when the concentration of substrate is higher than the concentration of enzyme, and in the case of steady-state, where the concentration of the complex enzyme-substrate is constant.
The Phoenix library contains four Michaelis-Menten models:
|
Model |
Description |
|
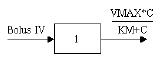
One-compartment with bolus input and Michaelis-Menten output |
|
|
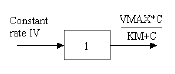
One-compartment with constant IV input and Michaelis-Menten output |
|
|
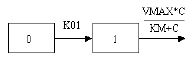
One-compartment with first-order input, Michaelis-Menten output and no time lag |
|
|
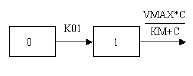
One-compartment with first-order input, Michaelis-Menten output and a lag time |
Michaelis-Menten models use constants to supply the dosing information. The required number of constants are listed below under each model number. For an explanation of dosing constants see “Dosing constants for the Michaelis-Menten model”.
Phoenix assumes that the time of the first dose is zero. For these models, times in the dataset must correspond to the dosing times, even if these times contain no observations. In this case, include a column for Weight in the dataset, and weight the observations as zero.
These models parameterize Vmax in terms of concentration per unit of time.
For a discussion of the difficulties in fitting the Michaelis-Menten models see Tong and Metzler (1980) and Metzler and Tong (1981).
One-compartment with bolus input and Michaelis-Menten output.
|
|
C(T) is the solution to the differential equation: |
|
Required constants |
Estimated parameters |
Secondary parameters |
|
Number of doses (N) |
V is the volume |
AUC=(D/V)(D/V/2+KM)/ VMAX |
|
Dose amount for dose N |
VM is the max elimination rate |
|
|
Time of dose for dose N |
KM is the Michaelis constant |
|
One-compartment with constant IV input and Michaelis-Menten output.
|
|
C(T) is the solution to the differential equations: |
|
Required constants |
Estimated parameters |
Secondary parameters |
|
Number of doses (N) |
V is the volume |
None |
|
Dose amount for dose N |
VM is the max elimination rate |
|
|
Start time for dose N |
KM is the Michaelis constant |
|
|
End time for dose N |
|
|
One-compartment with first-order input, Michaelis-Menten output and no lag time.
|
|
C(T) is the solution to the differential equation: |
|
Required constants |
Estimated parameters |
Secondary parameters |
|
Number of doses (N) |
V_F |
K01 half-life |
|
Dose amount for dose N |
K01is the absorption rate |
|
|
Time of dose for dose N |
VM is the max elimination rate |
|
|
|
KM is the Michaelis constant |
|
One-compartment with first-order input, Michaelis-Menten output and lag time.
|
|
C(T) is the solution to the differential equation: |
|
Required constants |
Estimated parameters |
Secondary parameters |
|
Number of doses (N) |
V_F |
K01 half-life |
|
Dose amount for dose N |
K01 is the absorption rate |
|
|
Time of dose for dose N |
VM is the max elimination rate |
|
|
|
KM is the Michaelis constant |
|
|
|
Tlag is the lag time |
|
Dosing constants for the Michaelis-Menten model
The number of constants in the model corresponds to the dosing route for the model.
Bolus and first-order input models require at least three dosing constants per profile.
|
Constant |
Description |
|
CON[0] |
Number of doses (N) |
|
CON[1] |
Dose amount for dose N |
|
CON[2] |
Time of dose for dose N |
|
CON[3] |
Dose amount for dose N+1 (if multiple doses are used) |
|
CON[4] |
Time of dose for dose N+1 (if multiple doses are used) |
Constant IV infusion models require at least four dosing constants per profile.
|
Constant |
Description |
|
CON[0] |
Number of doses (N) |
|
CON[1] |
Dose amount for dose N |
|
CON[2] |
Start time for dose N |
|
CON[3] |
End time for dose N |
|
CON[4] |
Dose amount for dose N+1 (if multiple doses are used) |
|
CON[5] |
Start time for dose N+1 (if multiple doses are used) |
|
CON[6] |
End time for dose N+1 (if multiple doses are used) |