This example demonstrates the general steps to prepare a CDISC dataset so that it can be used within Phoenix. The CDISC Data Preparer object produces analysis-ready datasets (SAMPLE and DOSE worksheets) in Phoenix, using CDISC formatted datasets as input. This example will also walk through submitting the output of the CDISC Data Preparer as input to an NCA object, which will be successfully executed.
There are steps common to many Phoenix objects (e.g., inserting an object, importing and mapping data to an object, and executing an object) and can be completed in multiple ways (e.g., main menu selection, right-click menu selection, drag and drop operation, etc.). For simplicity, only one mechanism is listed. Please refer to the Phoenix Functions chapter of the Phoenix Framework User’s Guide to familiarize yourself with the basic operations used in Phoenix and any alternative mechanisms available.
The sections in this example include:
•Setting up the project and data
•Setting up the CDISC Data Preparer object
•Mapping the results to an NCA model
Setting up the project and data
-
Create a new project named CDISC Prep Example.
-
Select File > CDISC > SDTM > Import.
-
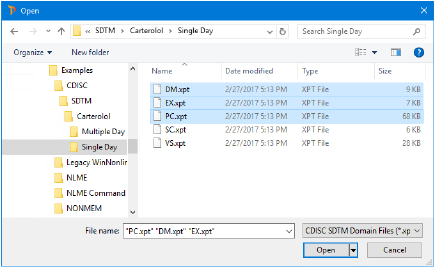
Import the DM.xpt, EX.xpt, and PC.xpt files from …\Examples\CDISC\Single Day.
-
Click Open.
-
Click Close.
The CDISC Data Preparer object is dependent on CDISC Navigator. The CDISC Navigator converts CDISC files, in SAS transport file format (.xpt), into Phoenix worksheets and verifies that the data conforms to the standards for the specified domain. Elapsed times are also converted from ISO format to numeric times in hours.
The CDISC SDTM Import dialog is displayed listing any problems that were encountered during the import and data validation processes.
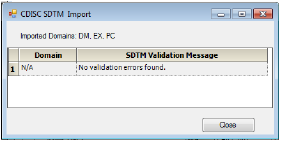
For this example, there were no errors.
Setting up the CDISC Data Preparer object
-
Select Workflow in the Object Browser and then select Insert > Data > CDISC Data Preparer.
-
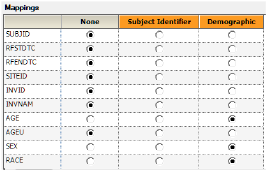
Drag the DM worksheet from the Data folder to the Demographics Mappings panel to map it as the input source.
The columns are automatically mapped to the appropriate contexts:
•USUBJID to the Subject Identifier context.
•AGE, SEX, and RACE to the Demographic context.These are the columns to include in the output datasets.
-
Select Exposure in the Setup panel list
-
Drag the EX worksheet from the Data folder to the Exposure Mappings panel.
-
Select PK Findings in the Setup panel list
-
Drag the PC worksheet from the Data folder to the PK Findings Mappings panel.
-
Map PCRFTDTC to the EXSTDTC context.
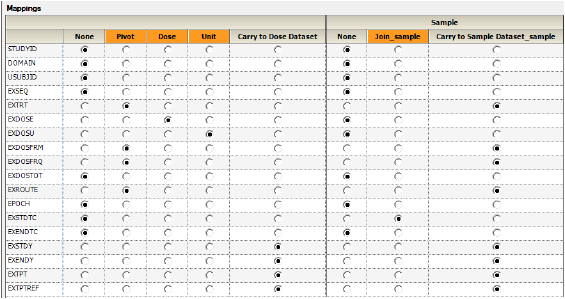
There are two mapping section in the Exposure Mappings. The first section specifies how exposure columns are treated when producing the Dose result dataset. The second section specifies which data columns from the Exposure domain are used to join to the Sample domain and which exposure columns to include in the Sample result dataset.
The columns are automatically mapped to the appropriate contexts:
•EXTRT, EXDOSFRM, EXDOSFRQ, EXROUTE to the Pivot context. These columns identify unique dose tests as defined in the Exposure tab.
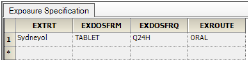
•EXDOSE to the Dose context. This column contains the actual dose amount.
•EXDOSU to the Unit context. This column contains the actual unit of the dose amount.
•EXSTDY, EXENDY, EXTPT, EXTPTREF to the Carry to Dose Dataset context.
•EXSTDTC to the Join context. This column will be used to match up data in the PK Findings domain.
•EXTRT, EXDOSFRM, EXDOSFRQ, EXROUTE, EXSTDY, EXENDY, EXTPT, EXTPTREF to the Carry to Sample Dataset context. This context enables sort keys to be specified between the dose and sample datasets during analysis.
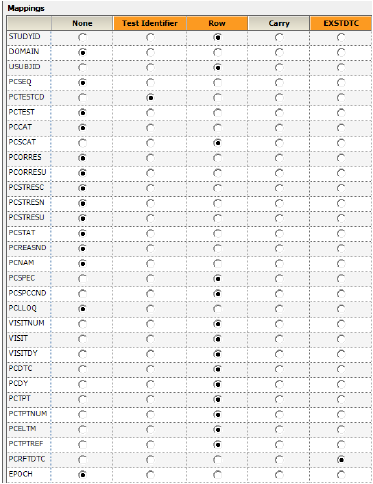
Most of the columns are automatically mapped to the appropriate contexts.
•PCTESTCD to the Test Identifier context. These columns are used to identify unique PK Findings tests. The unique tests are displayed in the PK Findings (Tests (PC)) tab in the properties.
•STUDYID, USUBJID, PCSCAT, PCSPEC, PCSPCCND, VISITNUM, VISIT, VISITDY, PCDTC, PCENDTC, PCDTC, PCDY, PCTPT, PCTPTNUM, PCELTM, PCTPTREF to the Row context. These are columns to include and represent the unique set of rows. All unique combinations of the Row and Join columns determine the number of sample rows in the result dataset.
Each column selected as a Join column in the EX domain will appear as a column mapping context for the PC domain. This allows specification of the columns in the PC domain that correspond to columns in the EX domain.
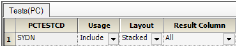
The PK Findings Tests(PC) tab on the Properties panel is used to select the tests to include, the layout, and the result columns to include in the result sample dataset.
In this example, the dataset only involves one test.
-
Accept the default settings for the SYDN PK Findings test, which are.
-
Usage set to Include to include the test in the output.
-
Layout set to Stacked to have the results presented in a stacked format.
-
Result Column set to All to have all results columns included in the output that match the CDISC specification for results columns in original, numeric, or text format.
Click  to execute the object. The Results are displayed on the Results tab. The CDISC Data Preparer object produces a Dose and Sample worksheet in the Output Data folder. A Log and Settings file are also produced. A Warnings and Errors file is generated if issues are encountered during execution.
to execute the object. The Results are displayed on the Results tab. The CDISC Data Preparer object produces a Dose and Sample worksheet in the Output Data folder. A Log and Settings file are also produced. A Warnings and Errors file is generated if issues are encountered during execution.
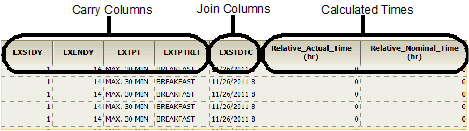
Dose
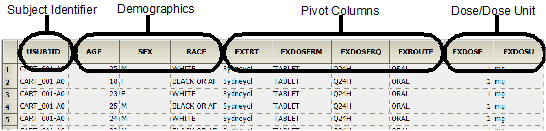
The Dose results worksheet contains the following columns in the order listed:
•Subject identifier columns
•Selected demographic columns
•Selected pivot columns
•Selected dose and dose unit columns
•Selected Carry to Dose Dataset columns
•Selected join columns
•Calculated relative actual time and relative nominal time columns
The following images show a portion of the Dose worksheet generated as part of this example.
Sample
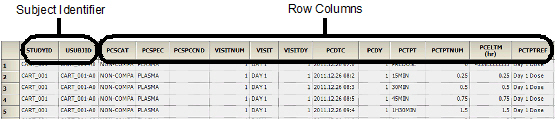
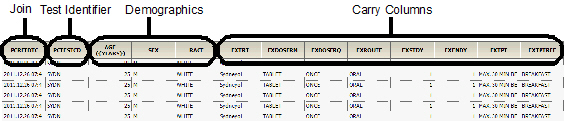
The Sample results worksheet contains the following columns in the order listed:
•Subject identifier columns
•Columns selected as Row in the PK Findings context mapping
•Selected join columns
•Selected test identifier columns
•Selected demographics
•Selected Carry to Sample Dataset columns
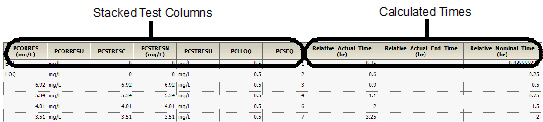
•PCORRES and PCRSTRESN columns for each unique unit in the source dataset for stacked tests
•Result column and result unit column for each pivoted test
•LLOQ column for each pivoted test
•If any results are stacked, PCORRESU, PCRSTRESU, and PCLLOQ columns containing the attributes associated with the stacked values
•Calculated relative actual time, relative actual end time, and relative nominal time columns
The following images show a portion of the Sample worksheet generated as part of this example.
Mapping the results to an NCA model
-
Select Workflow in the Object Browser and then select Insert > NCA and Toolbox > NCA.
-
Map the CDISC Data Preparer Sample worksheet to the Main input of the NCA object.
-
In the NCA Mappings panel click
 to open the Select Source dialog.
to open the Select Source dialog. -
Select the (+) next to CDISC Data Preparer to expand the list.
-
Select the Sample worksheet and click OK.
-
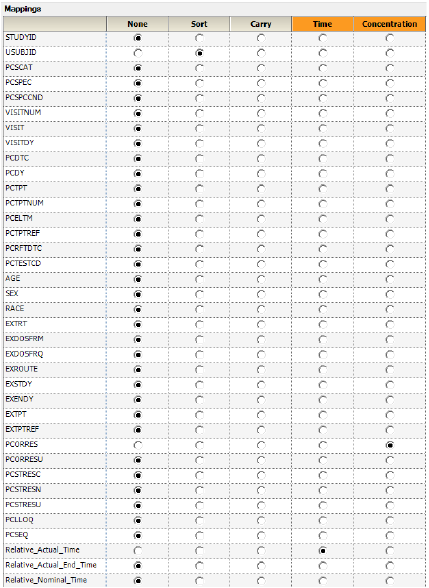
Use the option buttons in the NCA Mappings panel to map the following:
-
Map USUBJID to the Sort context.
-
Map Relative_Actual_Time to the Time context.
-
Map PCORRES to the Concentration context.
-
Map the CDISC Data Preparer Dose worksheet to the Dosing input of the NCA object.
-
Select Dosing in the Setup list.
-
In the Dosing Mappings panel click
 to open the Select Source dialog.
to open the Select Source dialog. -
Select the (+) next to CDISC Data Preparer to expand the list.
-
Select the Dose worksheet and click OK.
-
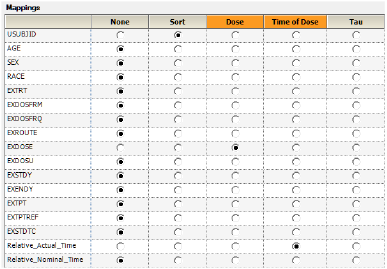
Use the option buttons in the NCA Mappings panel to map the following:
-
Map USUBJID to the Sort context.
-
Map Relative_Actual_Time to the Time context.
-
Map EXDOSE to the Dose context.
-
Click
 to execute the object.
to execute the object.
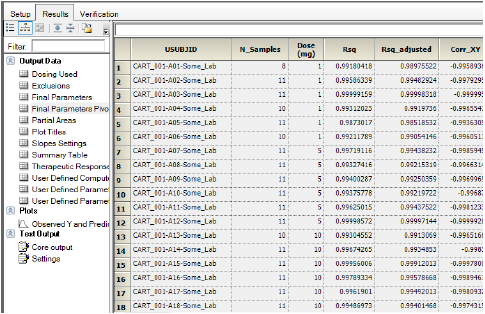
The results are displayed on the Results tab. The NCA object will produce worksheets and plots in the Output Data folder. It also produces a Core Output and Settings file. If any issues are encountered during execution, a Warnings and Errors file will be generated.
This example has shown how to use the CDISC Data Preparer object to convert CDISC data into a format that can be used by Phoenix. The object was set up and executed. The results of the CDISC Data Preparer were then mapped to another Phoenix object, NCA, as input. The execution of that NCA object was successfully completed without error.
