Sequential views allow a reviewer to see content initially broken down by sequence. These views are useful for QCing submissions on a more granular level. Sequential views include:
Sequence
Regulatory Activity
Origin
Sequence view displays all application sequences. The order can be modified by using the  icon to sort ascending or descending.
icon to sort ascending or descending.
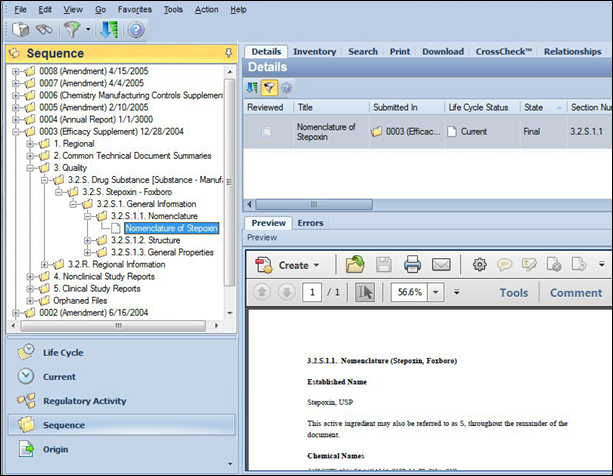
In this example, the sequence 0003 (an Efficacy Supplement) is selected and the Nomenclature of Stepoxin document is shown within section 3.2.S.1.1.
The Details tab displays the selected file reference Nomenclature of Stepoxin. The Preview pane displays the contents of the associated file.
The Regulatory Activity view displays each regulatory activity (such as an original application, supplement, annual report, variation, or line extension) grouped with its supporting submissions, such as amendments.
In the following example, in the Navigation pane, an Efficacy Supplement contains a file reference Nomenclature of Stepoxin within section 3.2.S.1.1.
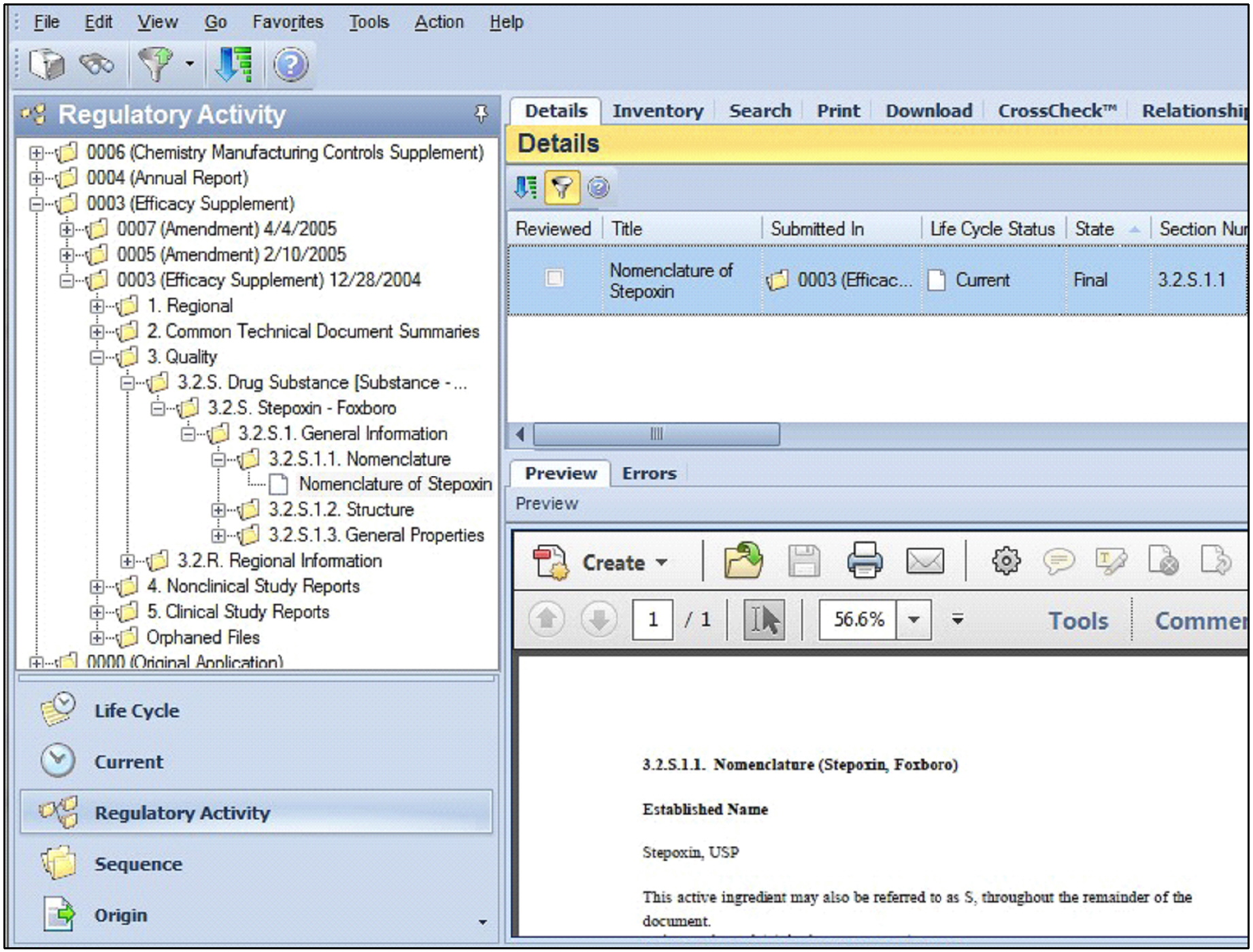
The Details tab displays the selected file reference Nomenclature of Stepoxin. The Preview pane displays the contents of the associated file.
Note that Regulatory Activity is highlighted at the bottom of the Navigation pane, indicating the view displayed.
Origin view displays sequence with all elements (sections and file references) in the exact order that was used in the published XML. It is set up similar to the Sequence view, however if CrossCheck is licensed, Origin view will also contain error codes and color-coordination to highlight validation errors contained within the documents or sections.
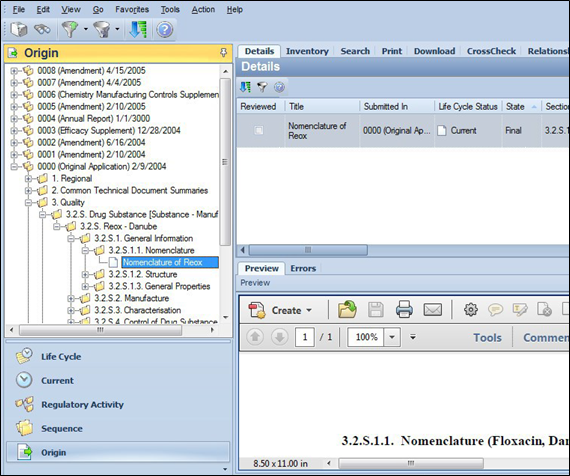
In this example, in the Navigation pane, an Efficacy Supplement contains a file reference Nomenclature of Stepoxin within section 3.2.S.1.1.
The Details tab displays the selected file reference Nomenclature of Stepoxin. The Preview pane displays the contents of the associated file.