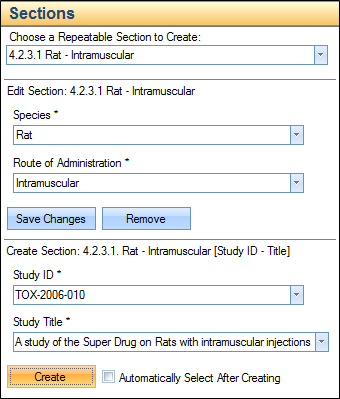
A specific set of information, or submission metadata is required to organize the studies, a study id and a study title. This can be used in combination with other properties, such as Species and/or Route of Administration, as shown in the screenshot below.
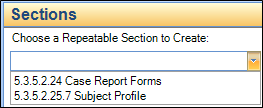
For studies without additional properties, simply click on the study type folder (for example, 4.3.1.3 Safety Pharmacology). When you choose the Sections tab, you will be able to enter the Study ID and Title as shown in the following:
The following screenshot shows a section/folder that was added to represent study TOX-2006-010 under the rat- intramuscular property group for 4.2.3.1 Single-Dose Toxicity studies.

For clinical studies, including Case Report Forms (CRFs) and/or subject profiles, additional sections may also be added for each study site where one or more CRFs or subject profiles originated.
Add the appropriate site sections by choosing the study and adding a repeatable section within the section for each required site as shown in the following image.
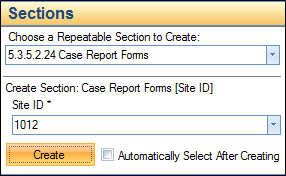
Or, click directly on the Case Report Forms or Subject Profile folder and create the site as shown in the following image.
Certain categories of studies require additional properties such as:
• 4.2.3.1 Single dose toxicity – Species and Route of Administration
• 4.2.3.2 Repeat dose toxicity – Species, Route of Administration, Duration
• 4.2.3.4.1 Long term [carcinogenicity] studies - Species
• 5.3.5.1 Study reports of controlled clinical studies pertinent to the claimed indication - Type of Control
For these studies, you must first supply the repeat section metadata as shown in the next image. These repeat sections are required to be able to add a study ID and study title.
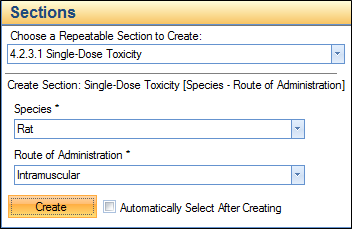
Completing this step will result in the generation of a new section in the TOC.
To create a study:
Select the section under which the study will appear in the tree.
Choose the Sections tab, for the purpose of this example, the selected section is 5.3.1.1, Bioavailability Study Reports.
If the study requires more properties, you will be prompted to add them.
• Once completed, select the newly generated section representing the property values that you chose that will appear underneath the study section.
Enter the Study ID and Title.
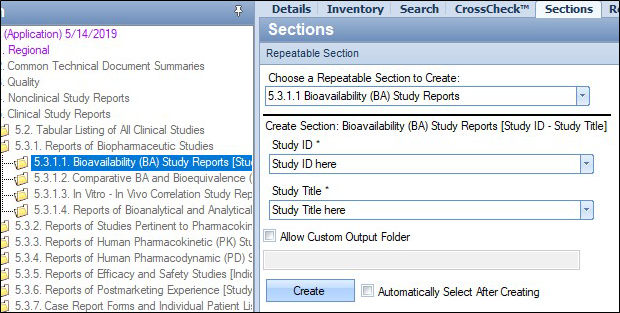
Click Create.
A new section will be created for the study, using the ID and Title.
If additional Studies are needed in different sites, repeat steps 1-5, where appropriate.
Modifying a Repeatable Section or Study
To modify a repeatable section or study, choose the repeatable section in the tree and select the Repeatable Section tab. Modify the properties, click Save Changes, and confirm your changes.
Note: For sequences in eCTD format, changing a repeatable section’s properties will only affect in- process sequences. Otherwise, a new repeatable section must be added.
If the sequence has been finalized or submitted using original/incorrect property values and a change is made using new sections, reviewers will see multiple sections instead of the single, corrected section.
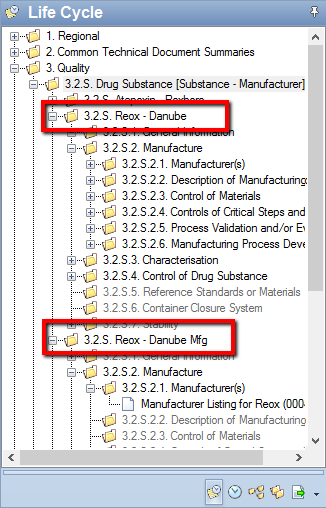
For example, if, in Sequence 0000, you used a drug substance manufacturer combination of Reox - Danube and later, in Sequence 0004, added the same substance, but a manufacturer of Danube Mfg, reviewers will see documents spread across two sets of 3.2.S sections, as shown in the following image, where 3.2.S.2 appears in both sections.