In order to meet granularity requirements for sections such as 3.2.S, 3.2.P, etc., specific sequence metadata must be added to PUBLISH to build out the structure per combination. To do so, perform the following steps:
Select the parent section from the tree.
In the Details pane, select the Sections tab.
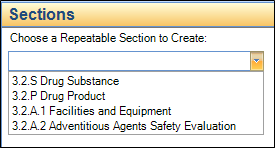
Choose the section to create from the dropdown list.
• This list will contain all repeatable sections that can be created at the level you have selected or in any subsections.
If there is only one possible repeatable section, this step will be skipped, and the section automatically chosen.
• The figure below shows the list of sections that can be created when you have selected Module 3 in the tree:
Note: If you do not see the correct section to create, ensure the correct parent section in the tree has been selected.
PUBLISH will prompt you to supply the properties for the selected section.
Required fields are marked with an asterisk (*).
For example, 3.2.S requires a Substance and Manufacturer, as shown below.
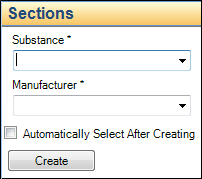
Note: Some fields are limited to values chosen from a list of controlled terms. If a required value is not in the list, contact your PUBLISH administrator to have the term added (see the “Adding Values via the Sections Properties Tab” section). In some cases, list values are dictated by a regulatory authority and cannot be added or modified.
After supplying all of the required properties for the new repeatable section, click Create to create the new section.
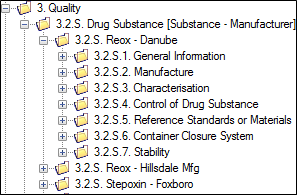
The new repeatable section will then appear as a section (folder), along with the required subsections.
The example below shows repeatable sections under 3.2.S for:
• Reox – Danube
Note that Reox - Danube has been expanded, showing the additional layers of granularity. The other two examples, when expanded will also show these subsections.
• Reox- Hillsdale Mfg
• Stepoxin - Foxborough
Note: You must add a repeatable section by supplying properties even if you will only be using the section once.
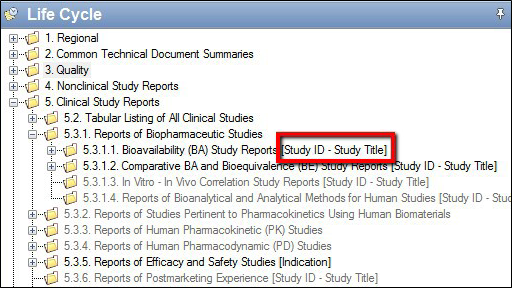
Clinical and Non-Clinical Studies
For US submissions, each study is added to the output via a separate XML called a Study Tagging File, or STF. During the publishing of a sequence, PUBLISH automatically generates a Study Tagging File in accordance with US FDA regulations using the sponsor-supplied metadata for the study.
For EMEA, SwissMedic, TGA and most Health Canada submissions, Study Tagging Files are not accepted. These regions use Section Extensions or Node Extensions to organize study data instead. Although the output structure may be slightly different, PUBLISH still takes advantage of the Sections tab, generating compliant Node Extensions based on supplied metadata to organize group studies in accordance with the specific CTD/eCTD guidance.
Detailed information on Sections Extensions can be found in the “Non-STF Study Node Extensions” section later in this guide.
GlobalSubmit uses the same concept of a repeatable section to manage the creation of each STF or Section Extensions, see the “Adding Application Metadata Repeatable Sections” section.
Repeatable Sections for additional information. As you can see below, studies use the same brackets to signify that sequence metadata must be added.